Cohesion
Cohesion stands for the internal strength of a material. It describes the binding forces between atoms and molecules that are active within a substance. The term cohesion is derived from the Latin word “cohaerere” (to be connected).
Cohesion in adhesives
In the case of adhesives, cohesion refers to the forces that create the internal cohesion of the adhesive. Throughout the entire bonding process, there must be an increase in cohesive forces in the adhesive or the adherend/glued joint in order to ensure a permanent connection of the bonded substrates.
Schematic structure of an adhesive bond
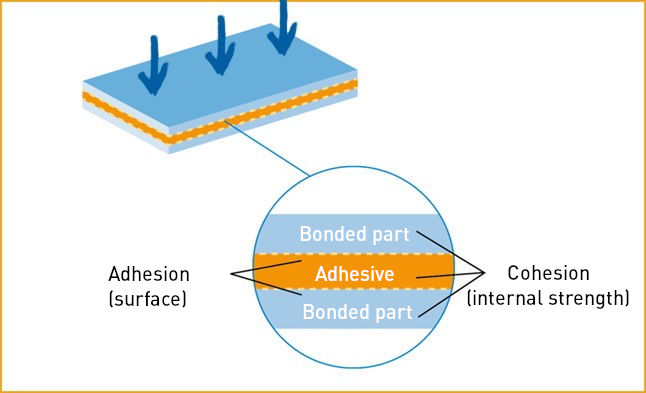
Source: IVK, Die Kunst des Klebens, Abb. 2
Cohesion building
The development of cohesion in the adhesive process can occur either through physical forces or through chemical cross-linking, which is why we speak of physically setting or reactive adhesive systems.
In the case of dispersions, cohesion is built up by “removing” the water content. For example, with white glue (PVAC), the wood sucks the water out of the adhesive. The polymer particles of the white glue intertwine, which is known as film formation.
The hot adhesives are liquid when applied, and the viscosity increases significantly as they cool down. In addition, cohesion further increases due to partial crystallisation in the adhesive.
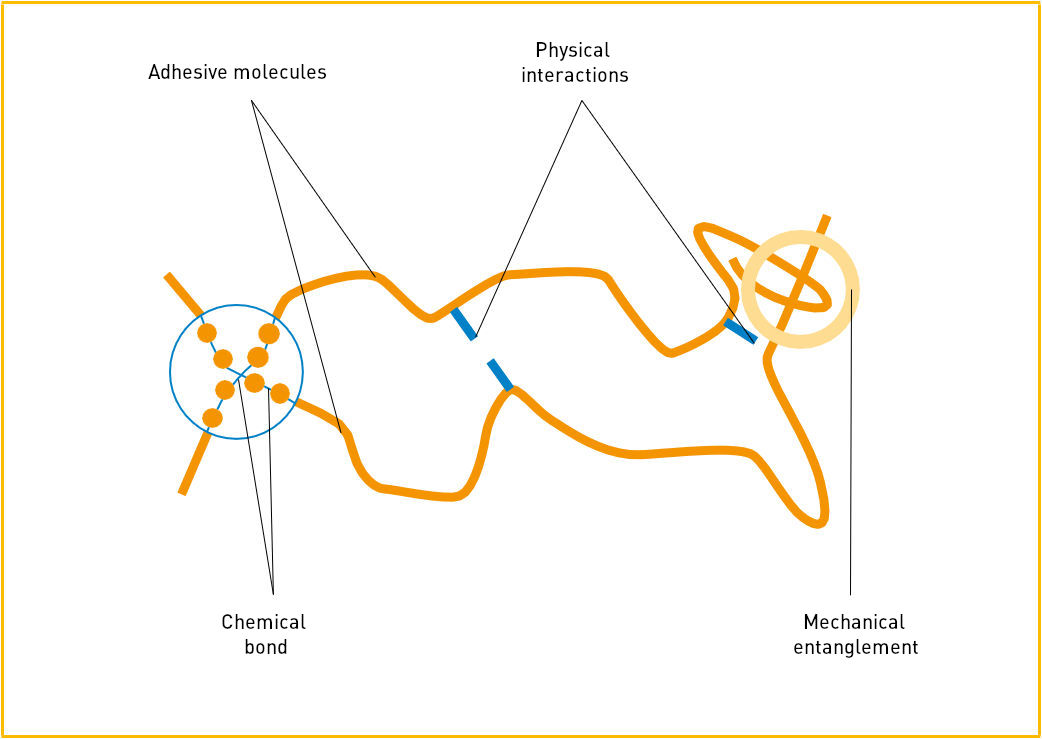
Source: IVK, Die Kunst des Klebens, Abb. 4
- The mechanical entanglement of thread-like macromolecules or matting of fibrous substances;
- The chemical bonds / cross-linking within molecules (reactive hot-melt adhesives);
- Physical interaction between the adhesive molecules, for example van der Wals forces or semi-crystalline structures
