What is surface tension?
Surface tension is an inward acting force (cohesion of the liquid) that occurs between liquids and adjacent surfaces of solids/gases.
The surface of the liquid behaves like a taut, elastic film.
In the vast majority of cases, the adhesive is in the liquid phase while it is being applied. The surface tension therefore also applies to liquid hot glue.
In order to achieve good wetting of the substrate with a hot-melt adhesive, the surface tension of the adhesive must be lower than that of the parts to be joined.
The adhesive must wet the surface of the substrate well. The wettability of the substrate is a prerequisite for a good bond.
The surface tension of the various substrates is very different.
It is a measure of liquids (adhesive) that measures the size of the force acting inward (cohesion at the interface to another phase) (air / substrates).
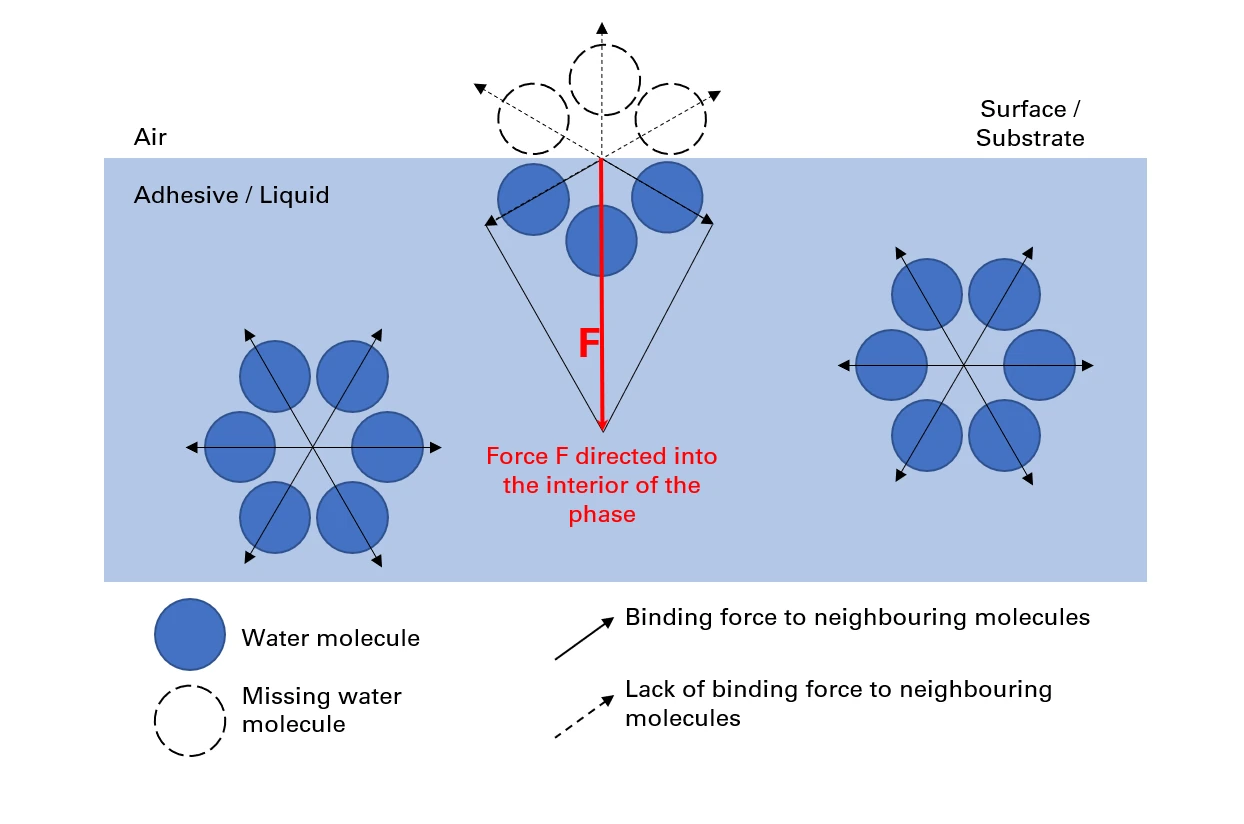
For this reason, surface tension is defined as “the work that must be done to increase the surface area of a liquid by one unit”.
